The Year in Review—How COVID-19 Transformed Our World
Reflecting on a challenging year in global health
As 2021 inches closer, many of us have begun our annual reflection, reviewing the events of the past year to gain perspective and shape the months ahead. Ordinarily, one might identify several themes that characterized the year—but in 2020, everything was filtered through the lens of the COVID-19 pandemic.
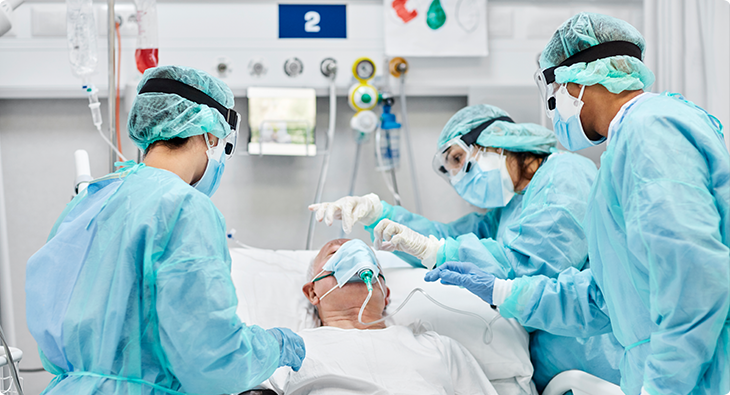
With tens of millions of cases and more than one million global deaths, the dramatic loss of life and complete societal and economic disruptions have created lasting changes in all of our lives. The pandemic changed how we work, interact with each other and our communities, teach our children, and measure our days.
Dedicating all available resources to address challenges
The pandemic also brought a beautiful side of humanity into focus: how we came together to fight for better health outcomes. At Luminex, this was a very personal lesson for us. Each day, we develop technologies and assays to improve clinical laboratory testing capabilities, and COVID-19 had a major impact on how we work together to quickly address time-sensitive challenges. In 2020, every member of the Luminex family worked diligently to develop, validate, manufacture, and support a host of new assays to facilitate SARS-CoV-2 detection and COVID-19 research.
Although developing these new solutions was an intense process, we knew our team was experienced, knowledgeable, and up to the challenge. We immediately recognized that building one assay wouldn’t overcome the enormous testing obstacles faced by labs around the world, so our goal was to develop a COVID-19 assay for every clinical testing platform in our portfolio. We wanted all Luminex customers to get the support they needed.
Creating a united front against COVID-19
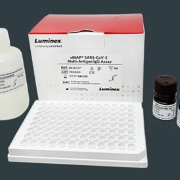
By early March, our internal efforts were well underway. Later that same month, we received our first Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA) for a SARS-CoV-2 assay as part of our ® SARS-CoV-2 Assay” href=”https://investor.luminexcorp.com/news-releases/news-release-details/luminex-receives-fda-emergency-use-authorization-ariesr-sars-cov”>ARIES® SARS-CoV-2 Assay, and then, we received authorization for our ® Technology Users Battle COVID-19 with New Serology Assays” href=”/blog/xmap-technology-users-battle-covid-19-with-new-serology-assays/”>xMAP® community mobilized quickly against the pandemic, deploying research partners and colleagues in other organizations as they engaged in COVID-19 research. We were also honored to support webinars featuring scientists from the Icahn School of Medicine at Mount Sinai and Rush University Medical Center to help others learn from their developments.
We won’t be sorry to leave 2020 in the past—and there are still challenges ahead as we move into the new year while continuing to fight the pandemic. However, even in the most difficult moments of this past year, we were struck by the dedication and tenacity of both our global workforce, as well as the clinical laboratory community at large, and we are honored to be part of the network of assay and technology developers supporting their unprecedented efforts.
We remain dedicated to supporting our customers and partners fighting the pandemic, which is why we have developed multiple flexible solutions for automating and facilitating SARS-CoV-2 testing and COVID-19 research needs. Click here to learn more about these solutions and stay informed on the most up-to-date COVID-19 testing information.
Related Content:
- Luminex Diagnostic COVID-19 Offerings [Webpage]
- How Luminex Is Supporting Research Partners’ Pandemic Response [Blog]
- The Versatility of Multiplex Antibody Titer Assays for COVID-19 and Beyond [Blog]
- ® Cookbook to Design Your Own Assays” href=”http://info.luminexcorp.com/en-us/research/download-the-xmap-cookbook”>xMAP® Cookbook to Design Your Own Assays [Download]