Bracing for Flu Season in a Pandemic World
How clinical laboratories can prepare as COVID-19 meets influenza

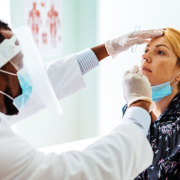
It’s officially autumn in the United States, which means pumpkin picking, college football, and, of course, the start of flu season. This annual occurrence is always a challenge for clinical laboratories, but this year, circulation of the influenza virus coincides with the ongoing COVID-19 pandemic, creating a new set of uncertain circumstances.
This situation is unique—flu season severity is largely unpredictable, and since we still have a lot to learn about SARS-CoV-2, healthcare experts aren’t sure how the two pathogens will interact. Meanwhile, labs and their vendors continue to face supply chain issues that could make it difficult to stock enough reagents, consumables, and test kits for a high-demand season.
Sherry Dunbar, PhD, MBA, Senior Director of Global Scientific Affairs has been working hard to raise awareness of the different challenges we should prepare for when the current pandemic meets flu season in the US.
To help the clinical lab community prepare for this situation, Dr. Dunbar recently published articles in Medical Laboratory Observer and Clinical Lab Manager. (And for clinical lab members, the MLO article offers Professional Acknowledgment for Continuing Education credits!)
Dr. Dunbar’s article in Medical Laboratory Observer describes both challenges and solutions for labs navigating flu season amid the pandemic. The highlights are summarized here:
- Labs will need more testing. “At a minimum, the pandemic means that clinical labs will not be able to rely solely on tried-and-true flu tests,” Dr. Dunbar writes. “Depending on local prevalence of COVID-19 infections, each lab will have to determine whether to add SARS-CoV-2 testing for all patients with respiratory symptoms, or whether they will need an algorithm to select which patients get tested for both flu and COVID-19.”
- Rapid molecular tests are recommended. The Infectious Diseases Society of America now recommends against using rapid antigen tests for influenza due to low sensitivity, and encourages the use of rapid molecular tests. “While these are typically not as fast as the 15-minute rapid antigen tests, they often produce results in just a few hours, which still allows healthcare providers to deliver same-day answers to patients along with guidance for the most appropriate treatment, if any,” Dr. Dunbar notes.
- Combination tests could ease supply-chain issues. Implementing a mini panel test covering influenza A/B, respiratory syncytial virus (RSV), and SARS-CoV-2 can reduce the total number of tests run, deliver results faster, and utilize fewer reagents and consumables. This mini panel test also enables co-infection case identification.
Although it’s difficult to predict how severe influenza will be each year, and there is still some uncertainty about how the pandemic will change this year’s flu and respiratory season, there are a number of ways clinical labs can prepare that may help reduce the burden on the healthcare system. Since there is significant overlap in symptoms for both COVID-19 and the flu, it’s important that labs prepare for an influx of testing as soon as possible, even if the flu season in the southern hemisphere was less severe than experts anticipated.
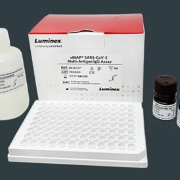
To support the detection of COVID-19 during the upcoming flu season, Luminex has developed a number of rapid molecular tests that detect SARS-CoV-2. One of these tests is the ® Respiratory Pathogen Panel” href=”https://www.luminexcorp.com/nxtag-respiratory-pathogen-panel/”>NxTAG® Respiratory Pathogen Panel, offers a comprehensive view of a patient’s health by detecting 21 common respiratory pathogens.
For more information about our additional SARS-CoV-2 testing solutions, check out our website!
Related Content
DiaSorin S.p.A. - Share Capital €55.948.257 R.E.A. 180729 - Fiscal Code and Subscription to Vercelli Companies Register no 13144290155