Precision Coupling with Molar Amounts of Protein
Precision coupling is useful for multiplex antibody assays, screening recombinant antibodies, and more!
I’ve been getting a lot of requests lately to provide more information on the molar coupling method. If you’ve been wondering how it works, here’s a quick look at how to incorporate molar coupling into your assay development.
When coupling whole immunoglobulins or antibody fragments for sandwich capture assays, coupling μg amounts of protein per million beads is the best way to optimize the amount of antibody needed to develop the most sensitive assay. However, to develop a multiplex assay for antibody titer or antigen specificity determination, it is best to couple antigens with the same moles per million beads. This is especially true when unrelated antigens of different molecular weights show some degree of cross-reactivity. By coupling with moles of protein per million beads, this promotes coupling the same number of molecules to bead surfaces, regardless of the protein’s molecular weight. For example, when coupling 1 μg per million beads of a 20 kDa protein and 1 μg of a 40kDa protein, the smaller protein will have twice as many molecules on the beads’ surface. This makes the signal a function of the number of molecules on the bead surface rather than reflecting antibody specificity.
To measure the effect molar coupling can have on determining antibody specificity, six antigens ranging in molecular weight from 24 kDa to 36.25 kDa were coupled at three different μg per million beads or pmol per million beads coating levels. Differences in signal were determined with 50X diluted sera specific for antigen D (Figure 1).
Figure 1. Comparison of Signal for Antigen D-Specific Sera with μg vs. Molar-Coupled Beads.
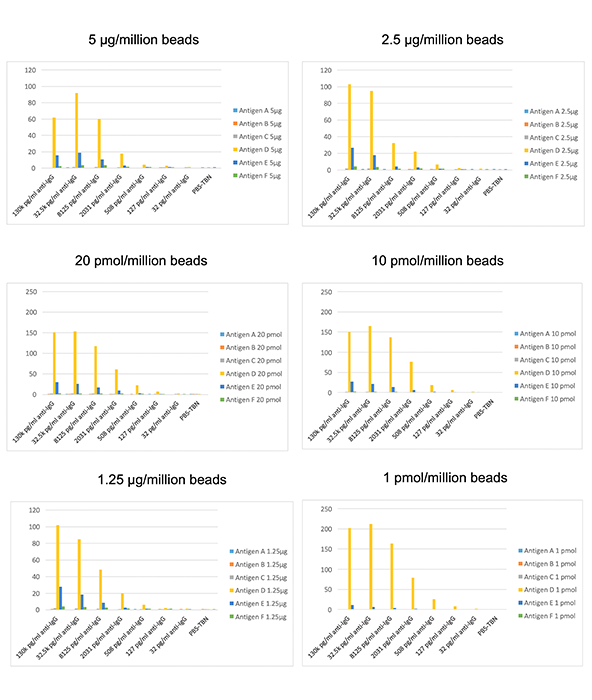
Beads coupled with 5 μg, 2.5 μg, and 1.25 μg/million beads showed good signal for antigen D as expected, but also high titers for several of the other unrelated antigens. By coupling at 20 pmol, 10 pmol, and 1 pmol, the specific titer for antigen D was significantly higher than the non-specific signal, which was decreased in comparison.
This coupling approach is also useful for screening recombinant antibodies for specificity, as well as for some protein-protein interaction assays. Excel-based workbooks for calculating how to prepare your protein stocks for standard coupling to beads or binding to MagPlex®-Avidin beads are available from your Field Application Scientist (FAS). Reach out to your FAS about how to use these tools, or for more information about molar coupling using xMAP® Technology.
Stephen Angeloni, PhD, is a Senior Field Application Scientist at Luminex.
Related Content
- Molar Protein Coupling Titration Calculations Template [Download]
- Avidin Bead Molar Protein Binding Titration Template [Download]
- Getting Started with xMAP® Technology [Video]
- Browse 1,200+ Partner Kits with xMAP® Kit Finder [Online Tool]
- xMAP® Cookbook to Design Your Own Assays [Download]
- View the xMAP Webinar Blog Series [Video]


