Medicaid Cuts and the Future of Respiratory Testing: Why Flexibility Is the New Imperative
By Stephanie Ibbotson
Healthcare’s new reality: How labs can stay resilient with smarter, more flexible testing approaches

The healthcare industry is once again facing a seismic shift. The passage of the One Big Beautiful Bill Act (OBBB) in July 2025 introduced sweeping changes to Medicaid and the Affordable Care Act (ACA), with $1 trillion in cuts projected through 2034, including $665 billion directly impacting hospitals.1 These changes are expected to reshape the financial landscape for clinical laboratories, hospitals, and diagnostic providers, especially those serving vulnerable populations.
What’s changing, and why it matters
The OBBB introduces:
- Stricter Medicaid eligibility requirements, including mandatory work verification.
- Reduced federal matching funds for hospitals serving Medicaid populations.
- Provider tax reforms that limit state flexibility in funding care.
- Expiration of ACA marketplace subsidies, potentially leaving millions uninsured.2
For clinical labs and hospitals, this means:
- Fewer covered patients and more uncompensated care.
- Reduced reimbursement for diagnostic testing.
- Increased pressure to optimize operational efficiency and cost-effectiveness.
Respiratory testing, already complex due to overlapping symptoms and seasonal variability, is particularly vulnerable. With declining reimbursement, tighter budgets, and shifting patient coverage, labs must rethink how they deliver high-quality diagnostics without compromising care.
The strategic response: smarter, more flexible testing
In this new reality, flexibility isn’t a luxury; it’s a necessity. Labs and hospitals need diagnostic platforms that:
- Adapt to changing patient volumes and payer mixes.
- Support diagnostic stewardship by aligning testing with patient population and clinical needs.
- Reduce waste and improve cost-efficiency.
This is where Diasorin’s comprehensive respiratory molecular testing portfolio, the LIAISON PLEX® Respiratory Flex Assay and Simplexa® Direct respiratory suite, can contribute to building a reliable and flexible testing approach.
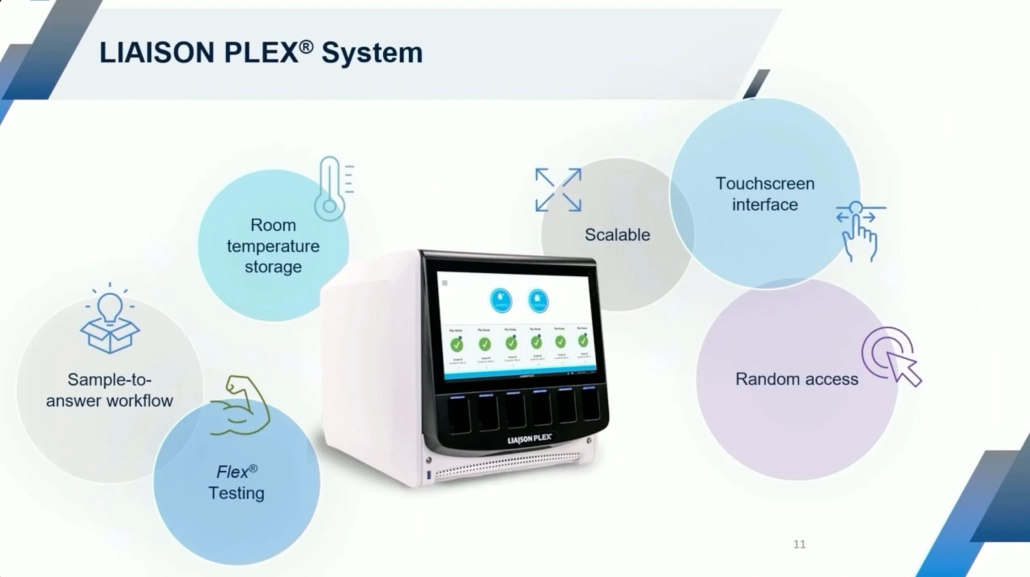
Introducing LIAISON PLEX®: a smarter way to test
The LIAISON PLEX® Respiratory Flex Assay is a sample-to-answer, multiplexed molecular test that detects up to 19 respiratory pathogens, including SARS-CoV-2, influenza, RSV, and bacterial targets, from a single nasopharyngeal swab.3
Key benefits for today’s healthcare environment
- Customizable Panels: Choose only the targets relevant to your patient population or clinical presentation. Pay only for what you report.
- Rapid Turnaround: Deliver results in under 2 hours with <2 minutes hands-on time, thereby streamlining workflows and reducing labor costs.4
- Diagnostic Stewardship: Align testing to institutional guidelines, seasonal trends and/or clinical presentations to support smarter care and better resource allocation.
- Cost-Conscious Design: Built for labs managing tighter reimbursement and budget constraints, especially in Medicaid-heavy regions.
- Adaptability without Sacrificing Quality: Create tailored panels that align with provider and network needs.
Simplexa®: targeted testing for critical needs
While LIAISON PLEX® offers flexibility for syndromic respiratory testing, Simplexa® (performed on the LIAISON® MDX) complements this approach by delivering rapid, targeted molecular solutions for high-priority pathogens.
- Respiratory Testing: Simplexa assays provide fast, accurate detection of key respiratory pathogens for labs that need streamlined workflows without large multiplex panels.
- Beyond Respiratory: Emerging pathogens like Candida auris, a multidrug-resistant fungus with high mortality rates, and congenital CMV, a leading cause of birth defects, underscore the importance of precision diagnostics. These conditions often affect vulnerable populations, including newborns, immunocompromised patients, and individuals in long-term care, many of whom are disproportionately impacted by Medicaid cuts.
- Flexible Testing Approaches: Multiplex platforms can support testing for these pathogens in specific sample types. However, a more targeted approach is often needed to enable early intervention, uphold stewardship principles, and manage costs.
- Simplexa Portfolio: Testing on the LIAISON MDX delivers that targeted capability, helping labs obtain clinically actionable results without unnecessary complexity or expense.
Conclusion: resilience through innovation
Medicaid cuts are reshaping the healthcare landscape, but labs and hospitals can adapt and thrive with the right tools and partners. Diasorin’s respiratory and targeted diagnostic solutions, LIAISON PLEX and Simplexa, offer future-ready options that balance clinical excellence with economic sustainability.
Let’s build your testing strategy together
At Diasorin, we understand that every lab and hospital faces unique challenges. That’s why our Market Access team is here to help you:
- Assess the impact of Medicaid changes on your diagnostic operations.
- Model cost-efficiency scenarios using LIAISON PLEX and Simplexa.
- Develop tailored testing strategies that align with your clinical and financial goals.
Whether you’re a rural hospital, a high-volume reference lab, or a health system navigating reimbursement shifts, we’re ready to partner with you.
Connect with our Market Access team by emailing MarketAccess@diasorin.com to explore how Diasorin solutions can support your testing strategy.
References:
- https://www.congress.gov/bill/119th-congress/house-bill/1/text
- https://www.hfma.org/fast-finance/regulatory-changes-hospitals-financial-impact/
- https://us.diasorin.com/en/molecular-diagnostics/kits-reagents/liaison-plex-respiratory-flex-assay
- https://www.captodayonline.com/fda-clears-diasorin-liaison-plex-system/